As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. As you go down a group electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus.

Electronegativity An Overview Sciencedirect Topics

Chemsolve Net Why The Dipole Moment Of Ch3f Is Less Than Ch3cl Although F Is More Electronegative Than Cl
Which Is The Most Electronegative Element Among Nitrogen Carbon Oxygen Boron And Aluminum Quora
Atoms whose anions are more stable than neutral atoms have a greater affinity.

Fluorine is more electronegative than chlorine. The only elements it doesnt vigorously react with are oxygen helium neon and argon. Fluorine is a chemical element with the symbol F and atomic number 9. The most electronegative atom fluorine is assigned a value of 4.
Sodium has an electronegativity of 09 while chlorine has an electronegativity of 30. It is two and a half times heavier than air. This has several implications.
Chlorine also reacts with sodium to create sodium chloride more commonly known as table salt. If B is a lot more electronegative than A then the electron pair is dragged right over to Bs end of the bond. The Electronegativity of Chlorine Fluorine and Oxygen.
The electron gain enthalpy of these elements becomes less negative upon moving down the group. Chlorine is a potent irritant to the eyes the upper respiratory tract and lungs. It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat andafter severe exposureedema filling with fluid.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. A value of 40 is assigned to fluorine the most electronegative element.
If it is closer to the nucleus the attraction is greater. Other highly electronegative elements are oxygen and chlorine. Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
In this case the hydrogen atom interacts with electronegative fluorine hydrogen or. Fluorine and water reaction is different from how other halogens react with water because fluorines electronegativity is much greater than other halogens. Fluorine the most reactive chemical element and the lightest member of the halogen elements.
Fluorines is the most electronegative element in periodic table Water and fluorine reaction will give products as below. But fluorine has the bonding pair in the 2-level rather than the 3-level as it is in chlorine. Among the elements fluorine ranks 24th in universal abundance and 13th in.
How did Rutherford figure out the structure of the atom without being able to see it. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Chlorine is a commonly used household cleaner and disinfectant.
It is one of the few elements that will form compounds with noble gases xenon krypton and radon. Chlorine is a chemical element with atomic. They kill bacteria and other potentially harmful microorganisms through a process known as sterilization.
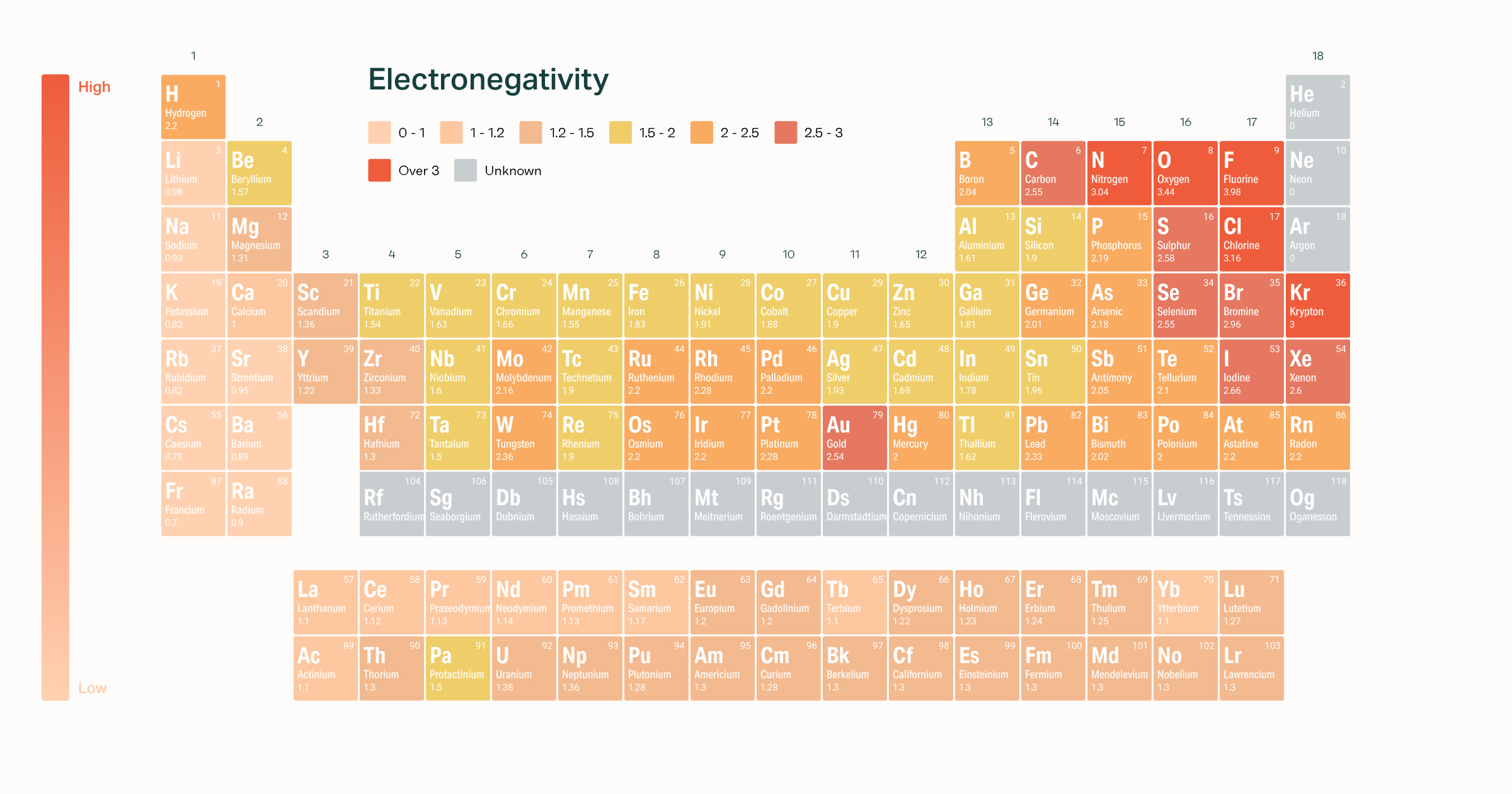
The difference between these values is 21 which means that sodium chloride has an ionic bond. The periodic table below shows the Pauling electronegativity scale. This is perhaps due to the fact that fluorine is the most electronegative element in the modern periodic table and therefore exerts the strongest attractive force on electrons amongst all the elements.
If one atom is overwhelmingly more electronegative than the other atom the electrons will not be shared and an ionic bond will result. Fluorine has an electronegativity of 398 on the Pauling Electronegativity Scale and a valence of 1A fluorine atom needs one electron to fill its outer electron shell and achieve stability which is why free fluorine exists as the F-ion. The electronegative difference between the atoms is greater than zero and less than 20.
Nonmetals are also less dense than metals and have lower melting and boiling points. The shared electrons spend more time close to the chlorine atom making the chlorine end of the molecule. The net pull from each end of the bond is the same as before but you have to remember that the lithium atom is smaller than a sodium atom.
In an ionic bond the more electronegative element will attract an electron from the less electronegative element. The electron pair is so close to the chlorine that an effective electron transfer from the sodium atom to the chlorine atom occursthe atoms are ionized. Both chlorine and bromine are used as disinfectants for drinking water swimming pools fresh wounds spas dishes and surfaces.
Why Fluorine Is the Most Electronegative Element. Moreover nonmetals have more positive affinity than metals. Now compare this with a lithium-chlorine bond.
Its chemical activity can be attributed to its extreme ability to attract electrons it is the most electronegative element and to the small size of its atoms. Chlorine - chlorine - Physical and chemical properties. Fluorine is the most electronegative element on the electronegativity chart followed by oxygen and then chlorine.
Chlorine is more electronegative than hydrogen by 096 electronegativity units. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. Fluorine is the most reactive and most electronegative of all the chemical elements.
The primary characteristic of nonmetals that makes them covalent is that they are highly electronegative which makes them more likely to form covalent bonds. Firstly it means that fluorine is always negative when combined with other elements. The hydrogen-chlorine bond in HCl or the hydrogen-oxygen bonds in water are typical.
For instance nonmetals are poorer conductors of heat and electricity than metal elements. Fluorine do not follow this trend. Chronic long-term exposure to chlorine gas in workers has resulted in respiratory effects including eye and throat irritation and airflow obstruction.
Fluorine is the most electronegative element. Chlorine most strongly attracts. All elements are compared to one another with the most electronegative element fluorine being assigned an electronegativity value of 398.
To all intents and purposes A has lost control of its electron and B has complete control over both electrons. Fluorine is the lightest halogen with atomic number 9. Now compare this with the lithium-chlorine bond.
Examples include most covalent bonds. The halogens exhibit high electro-negativity values. It becomes a liquid at 34 C 29 F.
The higher the associated electronegativity the more an atom or a. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Elemental fluorine is said to be the strongest elemental oxidizing agent.
Fluorine has lesser enthalpy than chlorine. Electronegativity is not measured in energy units but is rather a relative scale. Fluorine attracts electrons better than any other element.
Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core. Electronegativity symbolized as χ is the tendency for an atom of a given chemical element to attract shared electrons or electron density when forming a chemical bond. Thus fluorine is the most electronegative element while francium is one of the least electronegative.
As a result the shared pair of electrons will be closer to that atom. This strong attraction from the chlorine nucleus explains why chlorine is much more electronegative than sodium. No information is available on the carcinogenic effects of chlorine in humans from inhalation.
We can attribute it to the small size and the smaller 2p sub-shell of the atom of fluorine. Example molecules forming hydrogen bonding as a result of an unbalanced electrostatic potential. The large pull from the chlorine nucleus is why chlorine is much more electronegative than sodium is.
Electronegativity Of The Elements

Reduction Oxidation Chapter 14 And Oxygen Is The Most Abundant Element On Earth And Is Involved In Many Of The Most Important Chemical Reactions In Our Ppt Download

Account For The Following Observations Though Fluorine Is More Electronegative Than Chlorine Yet Bf 3 Is Weaker Lewis Acid Than Bcl 3 Snapsolve
The Chemical Bond The Atoms Of A Compound Are Held Together By Chemical Bonds Formed By The Interaction Of Electrons From Each Atom According To The Octet Rule Section 5 7c1 Atoms Bond Together To Form Molecules In Such A Way That Each Atom
The Lewis Acidity Of Bf3 Is Less Than Bcl3 Even Though Fluorine Is More Electronegative Than Chlorine It Is Due To

Quick Answer Which Of The Elements Below Has The Largest Electronegativity The Biggest

Fluorine Has Lower Electron Affinity Than Chlorine Because Of Youtube

Fluorine Is More Electronegative Than Chlorine Is The Statement True


